Bigotry: The Dark Danger
Miracles Within the Molecule

< <
5 / total: 6
Crystals: The Wondrous Order in MoleculesWhenever the word "crystal" is mentioned, people often think of a crystal vase or, rather more scientifically, the ice crystals in a snowflake. But crystal, in fact, is a flawless and magnificent work of art at the molecular level. The incomparable geometry in crystals amazed scientists when they were first discovered. The secret of their perfection was realized only very recently, as a result of the efforts of a great many experts. In order to understand Allah's geometrical artistry, we must first examine the three different states that molecules assume. Three Different States Alter any Molecule's Qualities
Most of us are aware of the three states of matter, of which the best-known example is water. In its normal room-temperature state, water is a liquid. When frozen, it assumes a solid form—ice—and when heated it turns into a gas we know as steam. Most molecules can assume these three different states of matter without losing their molecular structures. However, not all matter assumes these three different states. For example, if you heat gunpowder, you cannot obtain a gaseous form of it. Gunpowder explodes when heated and becomes a totally different molecule. Molten glass does not turn into a solid when chilled, it merely becomes hard—contrary to what is generally thought, a glass beaker is actually a liquid! 63 The reason why we assume that glass is s a solid is that it is so very hard and slow to flow. There is a thicker layer of glass at the bottoms of glasses and vases that have survived from ancient times because glass imperceptibly flows downwards—as can be seen in the ripples on old window panes. The substance we call "crystal" is a molecular structure displayed by substances in their solid form. To appreciate this better, consider that the gaseous, solid and fluid states of water possess the same molecular features. All are described by the formula H2O. In water's liquid form, its molecules slide over one another. In its gaseous state, they drift apart, independently from one another, over a wide volume. But in their solid state, the water molecules arrange themselves one after the other in a most symmetrical and immaculate order and crystallize. This is how ice forms. Any compound achieving a symmetrical form and geometrical order when it solidifies undergoes crystallization. If a compound cannot achieve this symmetrical order when it cools, then that compound is not a crystalline. That is one reason why glass is not regarded as a solid: The molecules that constitute it do not acquire a crystalline structure when cooled, and the arrangement of its molecules and atoms remains irregular. A substance that cannot achieve such an order cannot crystallize, for which reason they can never achieve a solid state. The Flawless Creation in Crystal
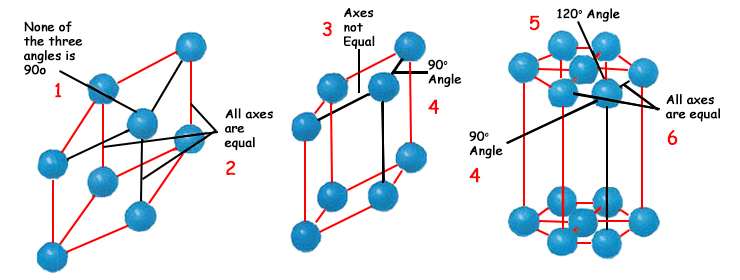
Atoms combine in various ways to form a three-dimensional molecule, which form is of great importance. As you have already seen, the functionality of the salt molecule is only possible through the sodium and chlorine atoms bound to one another attaining this three-dimensional form. The atoms and molecules that comprise a substance attain their most regular forms in the solid state. The three-dimensional forms they give rise to always have the same specific angles, which never change in any of the forms that the molecules form. So perfect is this order that not a single atom impairs the sequence and there is never a deviation in the angles between the atoms in a molecule. Atoms that combine at a 60-degree angle never combine at 61 degrees. If you heat this solid, making it liquid, then evaporate it and then chill it and freeze it again, the compound or element in question will re-assume the same perfect geometric form as before. The atoms will attach to one another literally as if they knew where they had to go, and the same exact angles will appear between them. When they recombine, no error will appear in the angles any greater than 1 degree. If the atoms formerly made a hexagonal prism when they came together, they will definitely do so again. Understanding the small scale in which this perfect order arises helps grasp the scale of this perfection. The diameter of an atom is approximately one hundred millionth of 3 centimeters (1.18 inch) and inside a 3 cm crystal contains 100 million x 100 million x 100 million atoms (100,000,000 x 100,000,000 x 100,000,000). If a regular progression of 1/1,000,000 of 3 cm (1.18 inch) is seen, then this substance may be regarded as a crystal. Therefore, every crystal possesses millions of regularly arranged atoms. 64 But you can't still see the scope of that order under the microscope. Thus no matter how many times you divide a solid substance—a metal for example—into fragments, you are still left with crystalline structures, because the atoms in even the smallest molecules still preserve their order. Even if you grind the substance into a powder, you are still left with crystal fragments. If you entirely melt this solid powder, then to a large extent it loses its crystalline structure.
The flat surface of a crystal is known as its face, and where two faces meet is the edge. The place where two edges meet is known as the corner. The edges surrounding a face generally form a simple geometrical shape such as a triangle or square. When all the surfaces that constitute the crystal are brought together, then cubic, rectangular or hexagonal prisms result. These structures can sometimes be very much more complex' and the greater the complexity of the structure, the greater the perfection of the emerging symmetry. Faces join to one another at every corner with perfect angles, which never exhibit an alteration or impairment. Prisms follow one another, and not even a flaw of 1/1,000 of a millimeter (that is, 4 x 10-5 of an inch) between geometrical shapes ever arises. The impairment of crystalline structure makes the substance assume an entirely different form, or else fall apart completely. Such an event would damage the entire order in nature, and would mean that a great many substances we are familiar with would be unrecognizable. In short, perfection must prevail within this order, and both perfection and order must be monitored at every moment. This, of course, reveals that everything created is under the protection of Allah. Even if different molecules are present in the same environment, their special crystalline structures prevent them never become intermingling with one another and thus losing their individual properties. For example, the salt and sugar crystals you throw into hot water soon dissolve into the liquid. Yet when you evaporate this solution, the salt and sugar in the water will crystallize separately and achieve their former structures. 65 The atoms in the salt never combine with one another at different angles, and the molecules never change their sequence. Indeed, any change in the sequence would result in the salt becoming an entirely different molecule.
How important are all this harmony and order? In this microworld—which we cannot see and of which most of us are unaware—how important is it for molecules to combine in a perfect geometrical arrangement while preserving their flawless angular values? Why is it they possess their own unique forms, and never lose them? If they did not possess these features, would the world really consist of random atoms and molecules? Of course, if Allah so wished it, no forms or geometrical arrangements would be necessary for the variety we observe around us to come into being. If He wished, there would be no need of either atoms or molecules to form matter. Allah's creation of this microworld in all its flawless complexity is based on most important wisdom. Even those who seek to deny Allah's existence cannot offer any explanation for this perfection and often express their astonishment at the creation in front of them. The presence of sublime artistry in even the smallest speck of dust proves that there can be no power outside that of Allah, Who reveals in the following verse: All praise belongs to Allah, the Lord of the heavens and the Lord of the Earth, Lord of all the worlds. All greatness belongs to Him in the heavens and Earth. He is the Almighty, the All-Wise. (Surat al-Jathiyya: 36-37) A Few Examples of Crystals' Flawless Structure
The minerals of which rocks are composed furnish the most familiar examples of crystals; everyone recognizes crystalline quartz, gems, and most semiprecious stones. But few realize that with few exceptions the entire solid crust of the Earth is crystalline. 66 Were you able to see this crust magnified, you would be utterly amazed at what you saw. You would see that everywhere you stepped was an uninterrupted plane bound with regular geometrical shapes, and you would realize that this was even more regular than everything else you see around you. You would realize that flawless structures, with their sublime symmetry and aesthetic appeal, are exhibited in even the smallest specks of dust, was also right under your feet. You would sense the splendor as well as the beauty of all this at every moment. In fact, with every step you take on Earth, you are faced with a perfect creation. What deceives you is merely the fact that you cannot perceive this sublime artistry with your naked eyes. Other familiar examples of crystals are snowflakes. The crystals that give rise to snowflakes are loosely bonded together, giving rise to such different patterns that no snowflake is identical to any other. On a snowy day, you can easily discover that snowflakes have very different shapes from one another by using a magnifying glass. The possibility of ever finding two identical snowflakes is exceedingly remote. Now, how many snowflakes fall in merely one acre over the course of a year? How about the mountains covered in snow and the sub-zero polar regions? Now, putting all these aside, consider how much snow falls on Earth over a year. If you had the means of bringing all these individual snowflakes together and examining them one by one, you would see that each one was completely different. The reason for this is the molecular property of the molecules constituting the snowflakes. Because of this, the snow crystals form with different geometrical structures, within their six-armed pattern.
The structures of all water molecules are basically the same, but these molecules can still exhibit variety. One out of every 5,000 water molecules may contain a deuterium atom instead of a hydrogen one. Additionally, in every 500 atoms there may be one oxygen atom with an atomic weight of 18 instead of 16. This difference causes a combination when ice crystallizes. There are 1018 water molecules in a single snowflake, and due to the variety of water molecules just described, 1015 of the water molecules forming a snowflake will be different from the others. According to this calculation, there is a 1 in 1024 possibility of two snowflakes having exactly the same sequence and shape. But the probability of such having occurred since the beginning of the universe is zero! 67 The really interesting fact is that all this infinite variety of snowflakes possesses a perfect, flawless symmetry. A snowflake is condensed water vapor that begins to form around a small dust particle, just a few microns in size. This microscopic shape is hexagonal, stemming from the structure of the water molecule. This crystal that forms grows increasingly larger, as small branches begin to grow from its arms. The colder the weather becomes, the faster these branches grow. As the flake is exposed to changes in the weather, capillary tubes begin forming on the emerging structure. As the snow spreads around and is subjected to different weather conditions this structural growth continues and starts to acquire a characteristic fitted to every condition. Since every branch in a single snowflake experiences identical growth, the branches all resemble one another, even though exceedingly complex structures emerge. In line with the original hexagonal structure, a symmetry based on the number six emerges, and the crystal acquires a three-dimensional structure. 68 These physical phenomena we have outlined, and the physical laws that give rise to them, are actually exceedingly complex. Therefore, just as snowflakes acquire very different shapes, they also acquire an immaculate symmetry, so finely calculated that it seems to have been designed on a computer. Most people never realize that just a single snowflake possesses exceptionally beautiful and aesthetic symmetrical shapes, and never come to understand its perfect structure. Nonetheless, a snowflake appears as an immaculate work of art because it is an example of Allah's artistry.
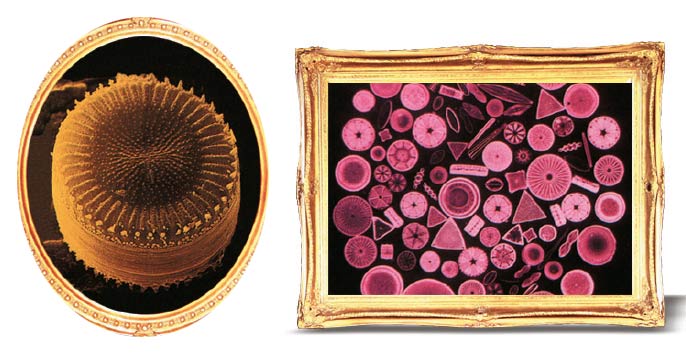
Another example of the marvels to be found in the crystal realm is a virus, which can lie dormant for hundreds of years and then miraculously come to life when it detects a living cell. The term "come to life" is a particularly apt, because viruses exhibit no signs of life until they sense the heat and moisture of a living cell. They have no organelles in the same way that single-celled organisms do. They only have a cell membrane that helps them to protect their DNA (and sometimes, RNA). They must hijack another living cell in order to replicate and make use of the shelter it offers.
Viruses can survive anywhere in the world, in heat or cold, in the air or underground, until they find a way of installing themselves inside a cell. The only reason why they don't break down and disintegrate is their crystal structure. This endows these tiny specks, invisible to the naked eye, with a flawless and symmetrical structure capable of protecting them. The distinct geometrical structures of viruses are the most evident features of their crystalline sheath. 69 Other microorganisms, not just viruses, also crystallize—a clear sign that microorganisms know the most far-sighted method of self-protection. When conditions grow difficult, various microorganisms such as algae and bacteria crystallize and enter into a kind of hibernation to survive. They remain in that state until conditions are better suited to them. As conditions worsen, each species of bacteria uses its own particular method of crystallization to rise up in the air. Its crystalline layer structure protects it against the harsh environmental conditions it may encounter among the clouds. When they encounter conditions better suited to themselves, these organisms lose the crystal structure protecting them and return to their behavior, feeding and multiplying. The formation of totally different, dazzling shapes with a flawless symmetry and immaculate geometry as a result of the combination of atoms and molecules—and the way that such a structures offer protection—are most important signs leading to faith. Everything you read about takes place at the molecular level. There is enormous care and incomparable intellect on Earth. This obvious truth pulls the rug out from under the feet of those who seek to ascribe other powers besides Allah and seek to deny His existence. His creativity reveals the hollow nature of their endeavors and strengthens the faith of believers. That is why such sublime artistry is displayed even in the tiniest details. As Allah reveals in His verses: Praise be to Allah, to Whom everything in the heavens and everything in the Earth belongs, and praise be to Him in the Hereafter. He is the All-Wise, the All-Aware. He knows what goes into the Earth and what comes out of it, and what comes down from heaven and what goes up into it. And He is the Most Merciful, the Ever-Forgiving. (Surah Saba': 1-2)
|
|||||||||||||||||||||
5 / total 6
You can read Harun Yahya's book Miracles Within the Molecule online, share it on social networks such as Facebook and Twitter, download it to your computer, use it in your homework and theses, and publish, copy or reproduce it on your own web sites or blogs without paying any copyright fee, so long as you acknowledge this site as the reference.