Bigotry: The Dark Danger
The Miracle of Protein

DOWNLOAD THE BOOK
CHAPTERS OF THE BOOK
< <
3 / total: 7
The Flawless Creation That Turns Inanimate
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 |
For example, one protein is keratin, the substance that forms the hard structure in hair, nails and feathers. Other proteins form a strong, nylon-like substance in the tendons that bind the bones to the muscles. Yet another protein, collagen, gives the skin its smooth elasticity and the bones their strength. Still another protein constitutes the elastic rubber-like tissue that surrounds the arteries. When light falls on the eye's retina, the protein rhodopsin initiates the process of vision. Other proteins make up the eye's transparent lens. Special transport proteins serve to help molecules enter and leave the cells. Without proteins, the DNA molecule—which encodes the data for all life—cannot be copied or preserve its information. In other words, proteins perform various tasks both within the structures of cells, the smallest units of life, and also in innumerable functions throughout the bodies of living things. Certain other proteins act as catalysts in order to speed up intracellular chemical reactions by up to billions of times. By working as a chemical team, they construct all the structural components of the cell. In addition to their construction abilities, they also break down large molecules in the cells into simpler compounds the cells can use. They permit the reactions to occur that provide the cells with energy. Also, special proteins in the muscle cells are necessary for the muscles to contract.
 |
|
|
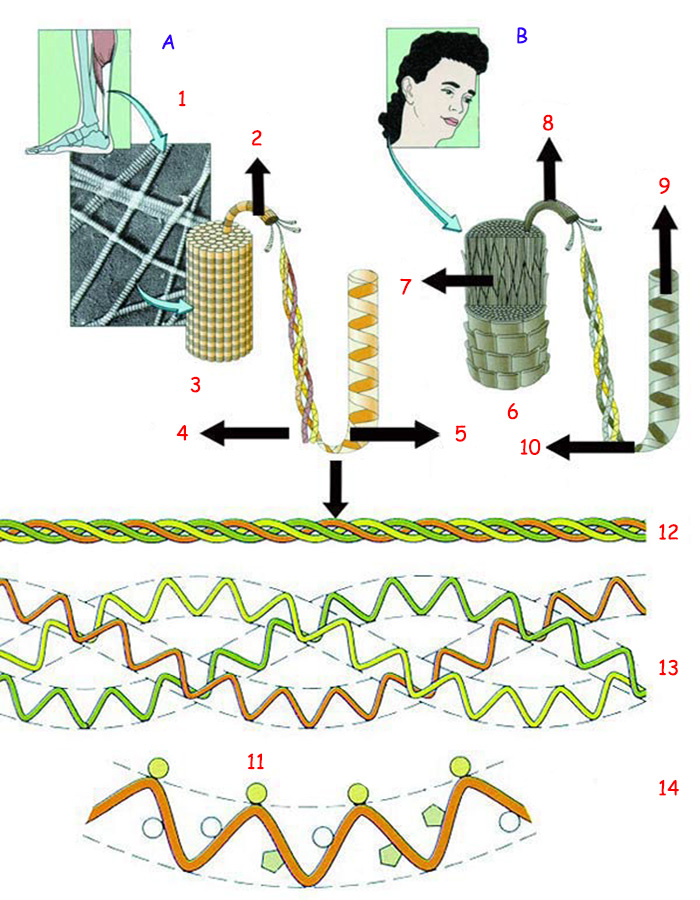
(A) Collagen, a strong fibrous protein |
(B) Keratin, the fibrous hair protein |
|
1. Tendon |
8. Keratin microfibril, |
|
Above you can see the structures of the protein collagen, which gives the bones their strength, and keratin, found in the hair. Below can be seen where the collagen fiber opens out. |
|
The listing above represents just a few of the thousands of varieties of protein. Even as you read these lines, every variety of protein in your body continues to work ceaselessly for you to enjoy a healthy life. Many needs, from your ability to read this book to being able to digest, and from the development of your body to your resistance to disease, are met through the proteins working constantly in your cells. The essential activities in all living things—not in human beings alone, but also in plants and all animal species down to the simplest bacteria, are based entirely on proteins.
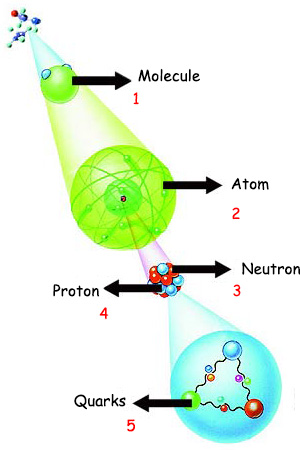 |
1. Molecule Specific atoms attach together in specific orders and with specific bonds, becoming miracle molecules like proteins, with specific functions. To the side, you see the internal structures of the atoms that compose a molecule. |
As this book will emphasize throughout, these miraculous molecules, the result of atoms combining in specific numbers and ways, work together in total harmony and fulfill unbelievable responsibilities by demonstrating the result of enormous intellect and consciousness. Every subject that we will consider from here on prompts an important question that every rational person of good conscience needs to ask: How are protein molecules—that arise from combinations of inanimate atoms, and which we might expect to lack any knowledge or competence—able to perform all these activities and display miraculous intelligence, organizational ability and a sense of responsibility? Everyone who reflects with true sincerity will understand that they are the flawless creations of Almighty and All-knowing Allah, and that all entities in the universe—from the greatest to the smallest—are under Allah's control and command. His dominion over all things is revealed in a verse from the Qur'an:
I have put my trust in Allah, my Lord and your Lord. There is no creature He does not hold by the forelock. My Lord is on a Straight Path.
(Surah Hud: 56)
Talented Proteins Built by Unconscious Atoms
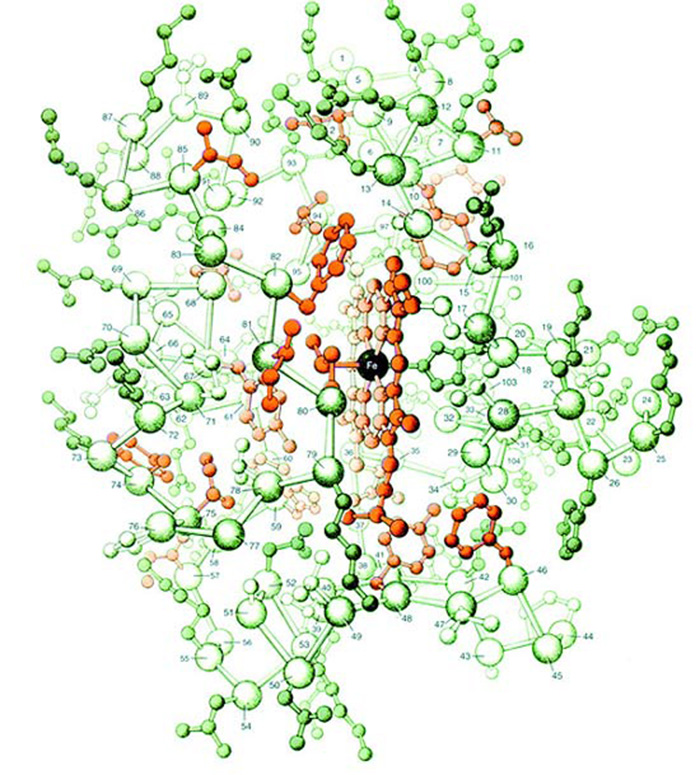
The diagram on the next page shows the atomic structure of the protein known as cytochrome-c. Just 5 millionth of a millimeter in size, this protein consists of approximately 1,000 atoms. As shown in the illustration, the organization and binding among these atoms is extremely sophisticated and complex.
Consider, now: Darwinists claim that these 1,000 atoms came together by chance and are bound to one another in the way you see. They also state that the protein cytochrome-c, with its vital functions for life, came into being as a result of these accidental combinations. Consider too that these 1,000 atoms include different elements such as iron, carbon and nitrogen atoms. In other words, the different atoms necessary to constitute cytochrome-c must be present all at once in a specific number and a specific place—and must then, as shown in the diagram, attach to one another by means of very different but appropriate chemical bonds. According to evolutionists' utterly illogical claims, all of this happened by chance, and a protein of the very greatest importance to life must have come into being in that unbelievable manner.
Furthermore, Darwinists also offer the same explanation for the origin of all the other thousands of proteins necessary for life. It is a violation of reason and logic to maintain that by combining in specific proportions and structures of inanimate atoms such as carbon, nitrogen, iron and phosphorus, devoid of any awareness of anything, gave rise to not just cytochrome-c but to all the proteins essential for life.
When you consider the tasks undertaken in the living body by these minute structures just 5 millionths of a millimeter in size, you can appreciate just how illogical and irrational it is to claim that unconscious atoms assembled such important structures by chance.
 |
|
Three-dimensional structure of the protein cytochrome-c |
Some proteins, for example, combine to form a substance that constitutes hair, nails and animal fur. Others comprise the tendons that connect muscle to bone. Moreover, proteins also carry the messages reaching the cells, and which receive and evaluate them. The "gates" and "pumping systems" that regulate entry into and departure from the cell are also proteins. Proteins also accelerate chemical reactions. The protein hemoglobin in red blood cells carries the oxygen to the tissues. The protein transferrin carries iron in the blood. Immunoglobulins are proteins that protect the body against bacteria and viruses. Fibrinogen and thrombin permit the blood to clot. Insulin is yet another variety of protein that regulates sugar metabolism in the body.
Other proteins are of great importance in the bodies of other living creatures besides human beings. The "antifreeze" protein in the blood of some fish protects ice crystals from forming in their tissues. The protein resilin possesses an almost perfect elasticity and thus permits the movement of insect wings. It's quite extraordinary how these molecules, which consist of only 20 amino acids—in other words, the combination of a few hundred atoms—can possess such different properties. It is definitely impossible for unconscious atoms to accidentally combine and by chance produce structures that can perform such important tasks, display intent, are able to organize and make the right decisions in the right place.
One matter to reflect on is how proteins consisting of more or less the same atoms can show such a wide variety of tasks and functions. When proteins' generally similar atoms are set out in different numbers and sequences, they endow a given protein molecule with different tasks and functions. It is impossible to account for this in terms of coincidence—a fact that Darwinists admit. About the formation of cytochrome-c, for instance, the prominent Turkish evolutionist Professor Ali Demirsoy has this to say:
In essence, the probability of the formation of a cytochrome-C sequence is zero... Otherwise, some metaphysical powers beyond our definition must have acted to form it, but to accept the latter explanation is not appropriately scientific. We thus must look into the first hypothesis. 3
In another chapter of his book, Demirsoy refers to the probability of cytochrome-C—an essential protein for life—forming coincidentally is "as unlikely as the possibility of a monkey writing the history of humanity on a typewriter without making any mistakes." 4
 |
 |
|
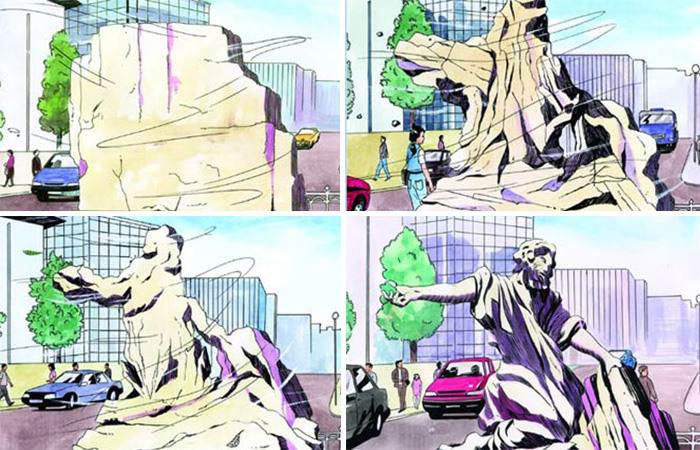
Coincidences cannever produce a superior, com plex model. To say that molecules such as proteins came into being by chance is even more illogical than claiming that a collection of rocks became a statue under the effects of random erosion, or that the waves beating on the seacost turned it into a marina. |
Since a monkey cannot type without making a mistake, the cytochrome-c protein can certainly not be formed by chance. However, as Demirsoy states in his first quotation, for Darwinists to accept the existence of supernatural forces is inappropriate. In other words, since the "scientific" objectives of evolutionist scientists are to deny the existence of Allah and support materialism, they are forced to accept that cytochrome-c came into being by chance. This claim is so illogical that even a little reflection lets you see the terrible error into which Darwinists have fallen. For instance, if someone claimed that powerful winds had turned a collection of stones in Trafalgar Square into a magnificent statue of a human being; or if someone said that powerful waves striking a cliff had produced the architectural façades in the red rock of Petra, Jordan, what would you think about that person's sincerity and psychological well-being? As you have seen, Darwinists are in such a logical impasse that out of all these impossibilities, they prefer the most unlikely of all. They close their eyes to evident truths, closing the door to their understanding and comprehension. It is plain for all to see that protein molecules were made for life by Allah, the Lord of Boundless Intellect, Knowledge and Power.
Flawless Systems in Line with Proteins' Duties
It is the order of their atoms that gives substances their characteristic features. The atoms comprising every substance, organic or otherwise, are arranged in specific groups known as molecules. From the book in your hand to the chair you are sitting in, from your own body to trees outside the window, everything is made up of atoms. However, animate and inanimate objects are differentiated from one another by their atoms being grouped and organized differently. In molecules that comprise the structures and systems of living things, the atoms have been ordered specially to enable life.
Protein is one of the four main groups of these organic molecules. (The others are nucleic acids, lipids and carbohydrates.) Again, the atoms in each molecular group are ordered differently. In this way, they acquire different properties and accordingly, undertake different functions.
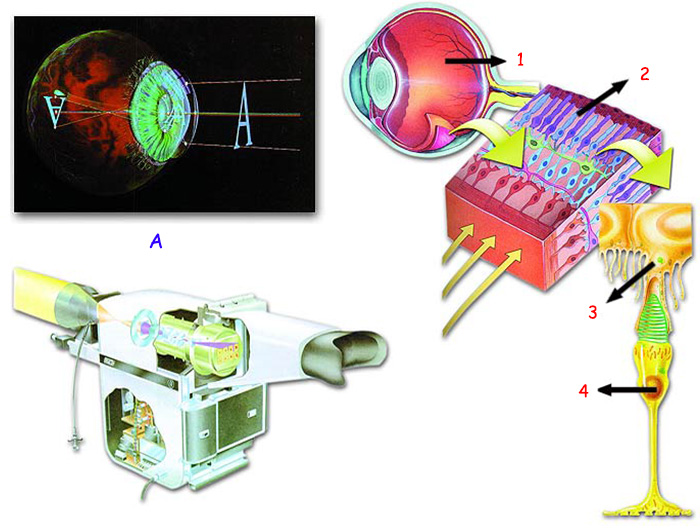
The order of the atoms is so sensitive and crucial that if the atoms of a single protein molecule fail to align themselves properly, this can cause irreparable damage to your body in a matter of moments. As an example, consider the phenomenon of vision. In the eye, which has a far superior technology than even the most advanced cameras, many proteins are involved in its ability to see. Just as in a camera, a number of components are responsible for the image to form. (However, there is clearly no possible comparison between the eye and the camera, whose components can never form as clear and as perfect an image as do the proteins in the eye.) A defect in any one of a camera's components will lead to either a defective image forming, or none at all. In the same way, if even one of the proteins in the eye fails to possess its correct molecular structure, vision may soon be impaired.
 |
|
|
A. The picture at the top left is a camera produced to imitate the human eye. (the top left of picture) |
|
|
1. Eye, |
3. Melanin proteins, |
|
The high-tech camera pictured above consists of hundreds of components. Imagine the best quality image you can obtain with it. Quite often, there will be blurring when your hand shakes. Inevitably that image's colors will never be exactly the same as they really appear. Now imagine the image that forms in your eye, consisting solely of proteins and fats. There is never any blurring or shake in that image. Neither does the focus ever go wrong. The colors are always accurate. It is a completely illogical to claim that unconscious atoms by chance began to transmit an image of such quality, which thousands of scientists, technical experts and engineers have been unable to reproduce. Clearly the eye was created, together with all its components, by a superior Creator. |
|
For example, the protein rhodopsin permits the eye to react to light. The slightest defect in the structure of rhodopsin will impair this process. Similarly, defects in the structure of proteins in the retina's cone cells (which enable the perception of color) will prevent the sufferer from being able to see in color. Another example is cataracts, which develop when the protein melanin is unable to protect the eye from the harmful effects of ultraviolet rays.
As you can see from these examples, proteins must possess the most appropriate molecular structures if they are to perform their essential duties. Therefore, it is equally essential that the amino acid molecules composing the proteins should also be in their ideal forms. Just as with proteins, detailed systems and flawless functions prevail in the structure of these amino acids.
The Order in Amino Acids
Proteins consist of molecules known as amino acids. Although smaller than proteins, amino acids still exhibit rather complex structures.
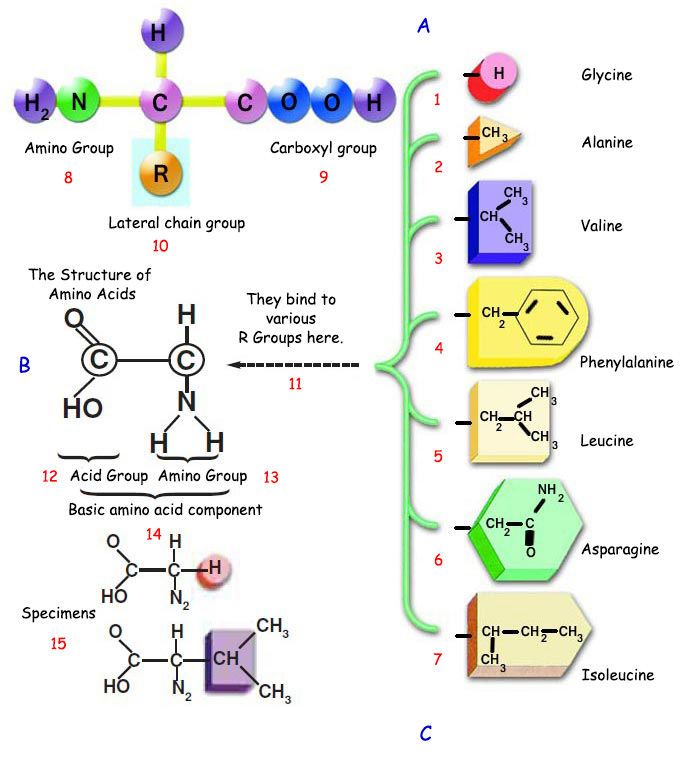
The atoms comprising amino acids fall into three separate categories: the amino group, the carboxyl group and the side chain or radical group. The amino and carboxyl groups are the same in all amino acids.
In the same way that various materials are used to produce a machine, there need to be components of various different properties in the protein "machines" if these are to perform their exceedingly complex functions in the body. In the side chain amino acids, the form, number and sequence of atoms, their electrical charges and diverse hydrogen binding capacities all endow the amino acids with considerable variety. And from this widely diverse material are produced widely different proteins. For instance, whether amino acids can dissolve in water or not depends on whether the side chain groups have a positive (+) or negative (-) electrical charge, or else no charge at all.
 |
||
|
A. Amino acids form through three groups of atoms, known as the amino, carboxyl and chain groups, attac hing to a carbon atom. |
||
|
1. Glycine |
6. Asparagine |
11. They bind to various R groups here. |
Amino acids with different properties line up alongside one another in different sequences, permitting the proteins that result to perform an astonishing range of functions in the body. However, the amino acids present in living structures are very special. Although more than 200 amino acids are found in nature, no more than 20 of them are found in proteins.
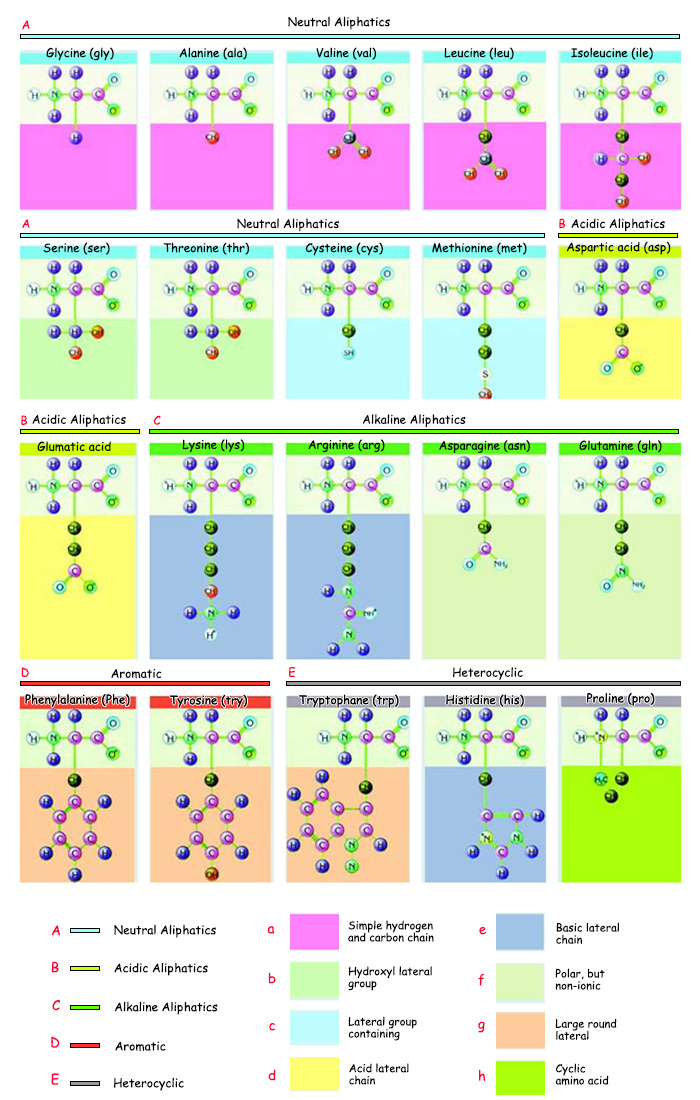
The 20 Varieties of Amino Acid in The Body |
|||
 |
|||
|
A. Neutral aliphatics |
a. Simple hydrogen and carbon chain |
e. Basic lateral chain |
|
|
1. Glycine (gly) |
6. Serine (ser) |
11. Glumatic acid (Glu) |
16. Phenylalanine (Phe) |
Why are Proteins Constituted of Only 20 of the 200 Amino Acids?
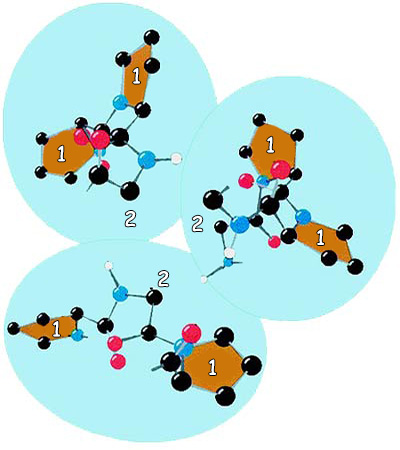 |
|
|
1. Pro, |
2. Gly |
|
The amino acid structure of the protein collagen is seen. As you see, one of each three amino acids is glycine(gly). Being very small, glycine is the most suitable amino acid for the structure of collagen. |
|
In theory, one would expect the number of amino acids in nature to be far more than 200. Even in human body, many amino acids not used in human proteins are used in the body's metabolic functions. Why, therefore, do proteins select only 20 amino acids when so many are more available?
We can answer this question by examining proteins' functions and structures. In order to perform their functions essential to life, proteins need to possess specific features, and amino acids are one of the main elements that give them those properties. For instance, it is essential that an amino acid possess hydrophobic (or water-repellent) side chains. But these side chains must not be very large, or else it will be impossible to pack and install them inside the proteins.
Side chains must also possess two features known as helix and layered formations. As a result of these, a protein can assume a three-dimensional form, and these are also essential for the protein to work properly.
Research has shown that of the 20 amino acids used in proteins, most are hydrophobic side chains. Half possess a-helix properties and the other half, b-layer properties.
Examine the properties of these 20 amino acids one by one, and you can understand why they have been specially selected for proteins. For instance, even glycine—the smallest and simplest amino acid—has a very important role to play in collagen, which is one of the most important proteins. If the three amino acids that comprise collagen, one is glycine. Its small dimensions play an important role in the structure of collagen, by permitting the chains comprising the protein to bind tightly together, which increases the resistance of the collagen fibers. Collagen fibers have been determined to have greater tensile strength than steel. If another side-chain amino acid were used in place of glycine, the resulting collagen fibers could not possess the same level of tensile strength. At the same time, were it not for glycine, the collagen fibers would also lack enough strength to bind cells to one another.
As you can see from this brief description, there is a consciousness and planning behind the selection of these 20 specific amino acids from among the 200 occurring naturally. Had this selection taken place at random, then the proteins necessary for life could never have formed. If only a single amino acid were any different from how it needs to be, a vital function would collapse, and life would therefore become impossible.
As you have seen, there are conscious systems, rational selection, and order in every phase of life.
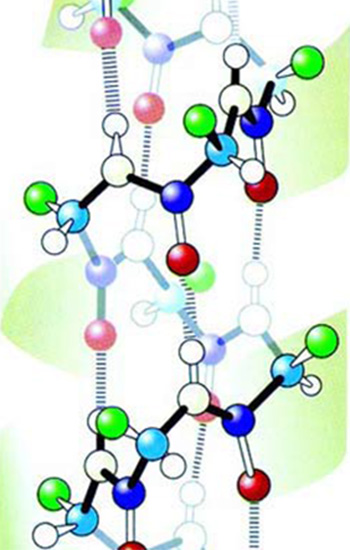 |
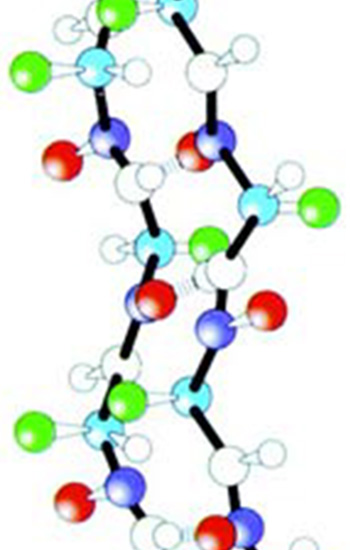 |
|
1. Side chain amino acid chain with helix. |
2. Side chain amino acid chain with layer. |
Proteins in Living Structures Are Formed from Left-Handed Amino Acids Only
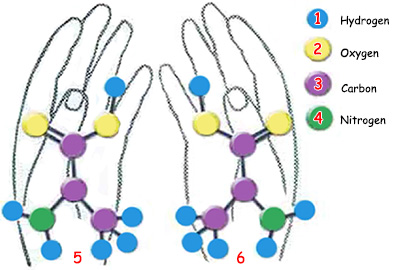 |
|
|
5. Left-handed amino acid |
6. Right-handed amino acid |
|
1. Hydrogen, |
3. Carbon, |
|
In nature there are two kinds of amino acids: right-handed and left-handed. |
|
As research has shown, it is not enough for amino acids to combine in different numbers and sequences to form proteins. All 20 of these amino acids must also be left-handed.
Of every amino acid found in nature, there are two different types: right-handed and left-handed. Each type is an opposite mirror image of the other, though all their other properties remain the same, just like right- and left-hand gloves.
The reason for this is that in one of the twin amino acids, a carbon atom binds to the amino group from the left and in the other one, from the right, which explains why the twin amino acids are called right-handed and left-handed. In nature, both types of amino acids are found in large quantities and in the same proportions. Each type of amino acid can just as easily form various compounds by entering into chemical reactions. In short, the only difference between the two lies in their different symmetry.
However, scientists discovered that the proteins in living things consisted only of left-handed amino acids. Not a single right-handed amino acid is found in any living structure.
More detailed studies discovered the important reason why the amino acids constituting proteins are all left-handed. Just like their left-handed counterparts, right-handed amino acids can combine with one another to form amino acid chains, but they prevent the resulting protein from assuming a three-dimensional shape. Yet—as you shall see in due course—in order for a protein to discharge its functions in living things, it absolutely must assume a three-dimensional form. It was realized that this being so, all amino acids had to be selected from among left-handed ones in order for a useful protein to emerge. The inclusion of even one right-handed amino acid would prevent the formation of a functional protein.
 |
|
It is as unlikely for all the amino acids making up proteins to be left-handed asit would be for a coin thrown into the air 10,000 times to always turn up heads. |
The revelation that only left-handed amino acids form the proteins in living things poses a major difficulty for Darwinists. As you have seen, in order for proteins to form, the selection consists of several stages. First of all, the 20 correct left-handed amino acids need to be selected from the more than 200 varieties in existence. A single incorrect amino acid becoming involved in the process—or a single correct but right-handed one—will make the protein functionless and redundant. The Britannica Science Encyclopedia, an outspoken defender of evolution, states that the amino acids of all living organisms on earth and the building blocks of complex polymers such as proteins all share the same left-handed asymmetry. This, it adds, is tantamount to tossing a coin a million times and having it always come up heads. The Encyclopedia claims that it is impossible to understand why molecules become left-handed or right-handed, and that this choice is fascinatingly related to the origin of life on Earth. 5
Inasmuch as Darwinists maintain that chance constitutes the origin of life, they cannot understand how random events should make such obviously conscious and well-directed choices. In fact, however, not blind chance but Allah, our Superior Creator, makes these conscious choices. In order to reject the fact of creation, Darwinists make irrational and illogical claims, suggesting that this selection is the work of "coincidences." According to their claim, the amino acids that comprise proteins—and the atoms that give rise to them—all accidently combined in the most appropriate manner to produce the proteins indispensable for life. No doubt, such a "scientific" claim exceeds the bounds of reason.
In fact, scientists estimate that the probability of a small protein being made up of left-handed amino acids alone is 1 in 10210. In mathematics, a probability of 1 in 1050 is regarded as zero. Since the number "1050" is obtained by writing 1 followed by 50 zeros, the likelihood of 1 in such a large number is therefore itself zero. That being so, it is even more impossible for any event with a probability of only 1 in 10210 (or 1 followed by 210 zeros) to actually occur. 6
The well-known chemist Walter T. Brown summarizes the impossibility of left-handed amino acids combining to form a single protein:
Each type of amino acid, when found in nonliving material or when synthesized in the laboratory, comes in two chemically equivalent forms. Half are right-handed, and half are left-handed—mirror images of each other. However, amino acids in life, including plants, animals, bacteria, molds, and even viruses, are essentially all left-handed. No known natural process can isolate either the left-handed or right-handed variety. The mathematical probability that chance processes could produce merely one tiny protein molecule with only left-handed amino acids is virtually zero. 7
The point here is that a conscious selection is taking place. Therefore, a conscious Will possessed of reason and information must be doing the "selecting." It's plain to see that this selection is performed by Allah, Who creates all living things within a given order, right down to their sub-atomic building blocks, and Who possesses a superior intellect, consciousness, knowledge and might. As Allah informs us in the Qur'an:
He directs the whole affair from heaven to earth ...
(Surat as-Sajda: 5)
The Plan in the Amino Acid Sequences
Fulfilling all the conditions described so far is still not sufficient for the formation of proteins. For every protein, a particular amino acid sequence is required.
Amino acids combine together like the links in a chain. As soon as they do, they assume a different shape and enable the protein to assume a three-dimensional form. As you shall see in detail later on, in order for proteins to fulfill their responsibilities, they must have a three-dimensional shape. But for this to be so, not a single amino acid can be deficient in any way or exchange its place in the sequence with a different amino acid. The absence or impairment of a single component will ruin the harmony of the whole and make the protein's structure inoperable.
Similarly, changing a single letter in a word can change that word's meaning or make it totally meaningless. For example, the word "grand" written with a t instead of d will produce the word "grant," which has a completely different meaning. If the letter a is omitted from "grand," then the meaningless "grnd" results. The same applies to proteins. A single amino acid changing its position will impair the protein "meaning" and make it unable to function. In fact, the protein thus altered will become an entirely different molecule, because every amino acid endows the protein with a particular property, just as a change of letter adds a different significance to a word. With its shape, electrical charge, and manner of entering into chemical reactions, every amino acid resembles a different letter.
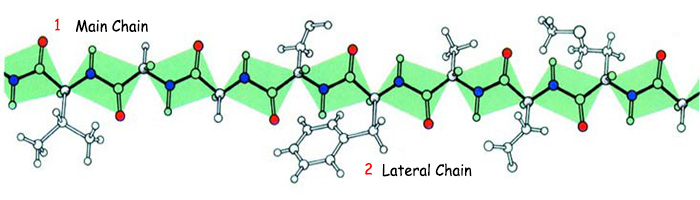 |
|
|
1. Main chain, |
2. Lateral chain |
|
An amino acid chain shown with aside chain. |
|
Mediterranean anemia, a genetic form of cancer, is an example of the kind of damage caused by the faulty or deficient writing of an amino acid. It is known that erythrocytes in the blood carry oxygen to all the cells in our bodies. The oxygen molecules are transported by the protein called hemoglobin, which is found in erythrocytes and consists of some 600 amino acids. A difference in just one amino acid in the structure of hemoglobin—if the amino acid known as glutamic acid is replaced by one called valine—gives rise to Mediterranean anemia. This one incorrect amino acid makes the hemoglobin protein unable to carry oxygen. When a mistake occurs in just one amino acid out of 600, a fatal disease results.
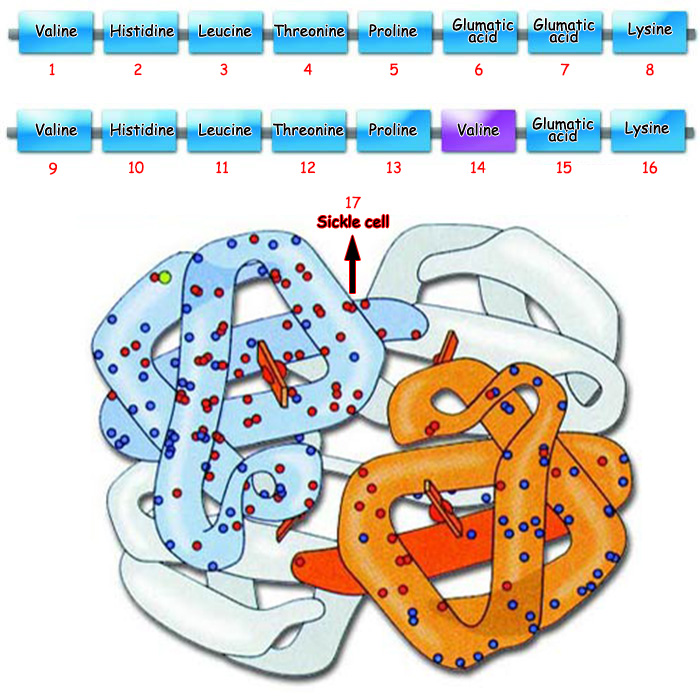 |
|||
|
1. Valine |
5. Proline |
9. Valine |
13. Proline |
|
Scikle cell anemia occurs when the amino acid valine replaces glutamic acid in the protein hemoglobin. The picture to the side shows a hemoglobin protein affected by sickle cell anemia. |
|||
But according to the theory of evolution, all these amino acids came together and arranged themselves by chance. As a result, various types of proteins emerged with thousands of beneficial and superior features and functions. Moreover, every one of these proteins "happens" to fulfill its duties accurately, without being redundant, and in coordination with all the others. It is clearly impossible for coincidences to establish any system that works with such immaculate order and displays such magnificent planning and programming. Coincidences can only give rise to disorder, confusion and chaos. They can never produce machines, products of advanced technology and a superior genius. Clearly, the fact that varieties of amino acid must be set out in a specific number and in a specific order in order to form useful proteins makes the Darwinist claim completely untenable.
This order belongs to Allah alone, Who created the atoms and molecules together with all the living things on Earth.
The Spesial Bonds That Join Amino Acids Together |
|
|
The various chemical bonds that join atoms and molecules are classified as ionic, covalent and weak. Covalent bonds hold together the atoms in amino acids, the building blocks of proteins. Weak bonds keep the amino acid chain in the three-dimensional form it has assumed through folding. Were it not for weak bonds, the proteins formed by the combination of amino acids could not assume their three-dimensional functional forms. In the absence of proteins, life would not bepossible. Interestingly, the temperature range that both covalent and weak bonds re quire is exactly that is found on Earth. Yet the structures and features of weak and covalent bonds are entirely different from each other. |
|
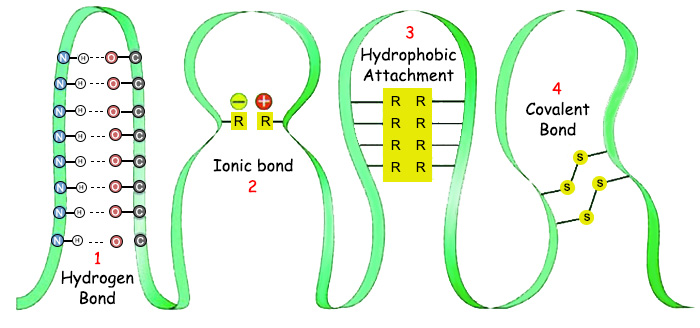 |
|
|
1. Hydrogen Bond, |
3. Hydrophobic Attachment, |
|
There is no natural rea son why they should both need the same temperature level. Nonetheless, both chemical bonds can be established only in the temperature range prevailing on Earth.If covalent bonds and weak functioned at different temperature ranges, then the formation of the proteins would again be impossible, because protein formation depends on these two chemical bonds being established simultaneously. If the temperature range for covalent bonds were not also appropriate for weak bonds, then proteins would not assume its final three-dimensional forms and would remain a meaningless, in effective chains. In the same way, if covalent bonds could not be formed at the same temperature as weak bonds, the amino acids could not combine and no protein chain could form. |
|
The Peptide Bond That Holds the Amino Acids Together
Another precondition must be met for proteins to form: In addition to their correct amino acids being in the proper sequence, they must be correctly bound to one another. This bond between amino acids is literally like a bridge. For each individual protein, the angles at which amino acids will be bound to one another on this bridge, their directions, and the variety and number of atoms within them have all been specially calculated. For example, if two amino acids are joined at an angle different than what it should be, this will prevent the completion of the bridge, and thus prevent the formation of the protein—resulting in an entirely different and useless molecule. These special bridges between amino acids are known as peptide bonds.
Scientists studying the biochemistry knew that almost all the atoms in the molecules in the structure of living things were connected by what's known as a covalent bond. However, researches revealed that amino acids combining to form proteins established a special bond previously undescribed. This is an unchanging rule for all proteins.
In 1902, Hofmeister and Fisher first uncovered the importance of these bonds in the formation of proteins. These two researchers performed a test in order to reveal the existence of this special bond.8 As a result, they determined the existence of a special bond occurring in proteins.
The most important characteristic distinguishing peptide bonds is that when moistened, they do not dissolve quickly. Peptide bonds can dissolve only at high temperatures when exposed to strong acids or bases for a long period. These peptide bonds allow proteins to be strong and resistant. In order for this special bond to be established a carboxyl group in an amino acid (in other words a special molecule containing carbon, oxygen and hydrogen atoms) must combine with the amino group in another amino acid (a special molecule containing nitrogen and hydrogen atoms). This establishes an important equilibrium at the connection points along the protein chain. During the formation of these bonds, water is released which constitutes up to 80% of protein molecules.
At this point, you may well ask: While the molecules of all the living things on Earth are joined by a covalent bond, what permits the peptide bond among amino acids?
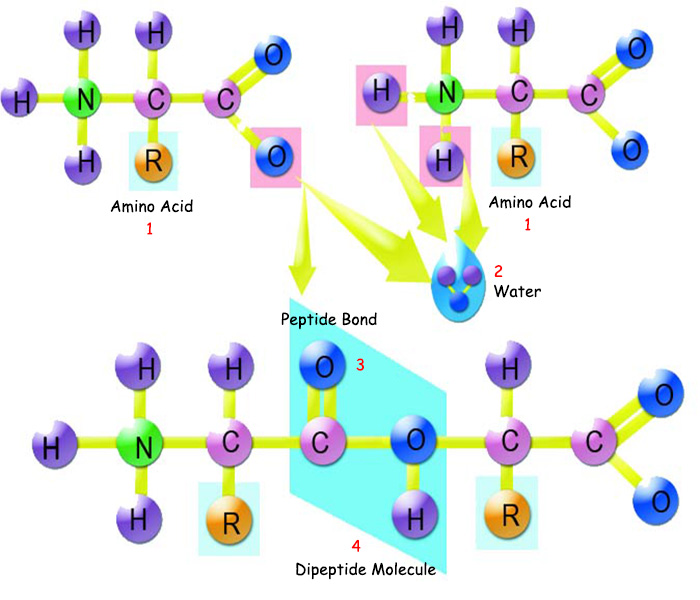 |
|
|
1. Amino Acid |
3. Peptide Bond, |
|
Amino acids attach to one another by peptide bonds. The main feature that distinguishes them from other bonds is that peptide bonds do not easily dissolve, making proteins strong and resistant. |
|
Research has shown that when amino acids combine, only approximately 50% of the bonds that form among them are peptide bonds, the others being attached to one another by other bonds. When attached by these different bonds, no protein molecule emerges.9 Just as specific varieties of amino acids must be arranged in specific amounts and in a specific sequence, with each one being left-handed, in order for a protein to form, also there needs to be a peptide bond between them. If just one of these conditions fails to be met, then protein cannot form.
Remember that an average protein molecule contains several hundred amino acids. The odds of any amino acid being attached to another one by a peptide bond is one in two, or 50%.
To summarize what features the amino acid chains must possess for a single protein to form:
1. Of the more than 200 varieties of amino acid in nature, only 20 are found in living organisms. The requisite ones for the protein to be made need to be distinguished and selected from these 200 amino acids.
2. The selected amino acids must all be left-handed, not right-handed.
3. After the proper amino acids have been selected in the correct amounts, they need to be arranged in a particular sequence for protein to be formed.
4. After arranging in the correct sequence, the selected amino acids must be joined together with a peptide bond.
It's clearly impossible to account for even one of these conditions for the formation of a single protein in terms of chance. Therefore, it is completely out of question for several conditions, none of which could have occurred by chance, to combine together (again by chance!) and give rise to a protein.
Molecular biologists have carried out a great many probability studies on the impossibility of proteins forming by chance. These include such well-known scientists as Harold Morowitz, Fred Hoyle, Ilya Prigogine, Hubert Yockey and Robert Sauer. Despite being Darwinists, they have concluded that there is no chance at all of macromolecules like proteins coming into existence spontaneously.
|
Let us imagine that the letters that comprise this sentence are the amino acids that constitute a protein. There is no chance of the letters in this sentence forming a meaningful sentence if they are distributed at random. Such a random action will produce billions of different outcomes. Just three of these possibilities are as follows: |
|
1. First and foremost, some of the letters will fall face down. |
|
2. Some of the letters will fall on their sides or upside down. Moreover, the letters may not line up side by side when they are thrown down. Even if we assume they line upside by side, some will form an oval shape and others a circle. |
|
3. The chances of them lining upside by side are very small. Even if we assume they do line up side by side, against all the odds, they will still be in the wrong order. And the result will be a mass of letters signify ing nothing. As you see from this example, if the amino acids in nature come together by chance, some will be right-handed and others left-handed. When set out at random, they will form a meaningless sequence. Thus no protein will emerge. When you read a meaningful sentence, you can be sure some rational, informed human being wrote it. In the same way, proteins that have existed for billions of years show the existence of a superior Creator Who brought them into being with His intellect and consciousness. |
Through a mathematical calculation, you can see for yourself the impossibility of a small protein molecule, 100 amino acids long, coming into being by chance:
The chances of all 100 amino acids in a protein being left-handed as a result of coincidence is approximately (1/2)100, or 1 in 1030. Since there are 20 amino acids in the proteins of living things, the probability of obtaining a special amino acid in any given region of the amino acid chain is 1/20. The probability of obtaining a special protein 100 amino acids long is (1/20)100 or 10130. The odds of obtaining a peptide bond in any particular amino acid chain are approximately even, or 1 in 2 (50%). The probability of obtaining a 100-amino acid chain in which all the bonds are peptide is approximately (1/2)100 or 1 in 1030—a probability so small as to be non-existent.
Now, bearing in mind all these probability calculations, let's compute the likelihood of a chain in which all the bonds are peptide, in which all the 100 amino acids are left-handed, and in which the amino acids are arranged in the proper sequence for a particular protein coming into existence by chance. That probability is approximately 1 in 10190. Even if we allowed a period as long as the age of the Earth for such an event to occur, in practical terms there is no chance of its happening. Moreover, if you recall that in mathematical terms, a probability of 1 in 1050 is zero, we can see that no such thing can ever take place. Indeed, considering that the number 10190 actually contains four 1050s, the impossibility becomes even more apparent (1050 times 1050 times 1050 times 1040 = 10190). In the light of these findings, the world's famous biochemist Michael Behe has stated that the probability of a protein 100 amino acids long being obtained is even less than that of being able to find a marked grain of sand in the Sahara Desert (which is 8.6 million square kilometers in size) with one's eyes closed. 10
Given that it's totally impossible for even a single protein to come into being by chance, it's evidently illogical to claim that all the thousands of varieties of functioning proteins in living structures could have formed by chance and given rise to cells. In addition, it is not only proteins that make up the body of a cell. The cell also consists of other organic molecules created with a superior consciousness, and are organized with that same matchless planning.
 |
|
Professor Michael Behe has stated that the odds of obtaining an appropriate sequence in a protein 100 amino acids long are even smaller than those of finding a marked grain of sand in the Sahara Desert with your eyes shut. This example alone is anindication that proteins were created by Allah. |
Every stage of protein formation reveals the presence of consciousness, information, will, intellect, power and planning. These features belong to our Lord, a Superior Creator. Those who believe in the creative powers of other entities apart from Allah—or of chance, which is helpless and lacks the power to create anything—make a terrible error and have gone badly astray.
In one verse Allah reveals:
He to Whom the kingdom of the heavens and the Earth belongs. He does not have a son and He has no partner in the Kingdom. He created everything and determined it most exactly. But they have adopted gods apart from Him which do not create anything but are themselves created. They have no power to harm or help themselves. They have no power over death or life or resurrection.
(Surat al-Furqan: 2-3)
The Four Different Structures of Proteins
The physical, chemical and biological properties of proteins, and the resulting functions they perform, determine the type of amino acids in their structures, their sequence, and the arrangement in these amino acids' side chains.
Proteins may have a primary, secondary, tertiary, or quaternary structure.
A primary structure emerges from straight amino acid chains. A proteins in a primary structure is not functional, but when added to one of secondary, tertiary or quaternary structures, it may play a role in bodily processes. The secondary structure forms with the long amino acid assuming a spiral form. Proteins such as actin, myosin, fibrinogen, keratin and b-keratin exhibit a secondary structure. Proteins with a tertiary structure emerge within the amino acid chain folds and bends, resulting in a structure reminiscent of a ball of wool.
The quaternary structure emerges from two or more amino acid chains of equal or different length.
Detailing the features of these different structures and the functions they bestow on proteins can help you see the superior creation with which these molecules were brought into being.
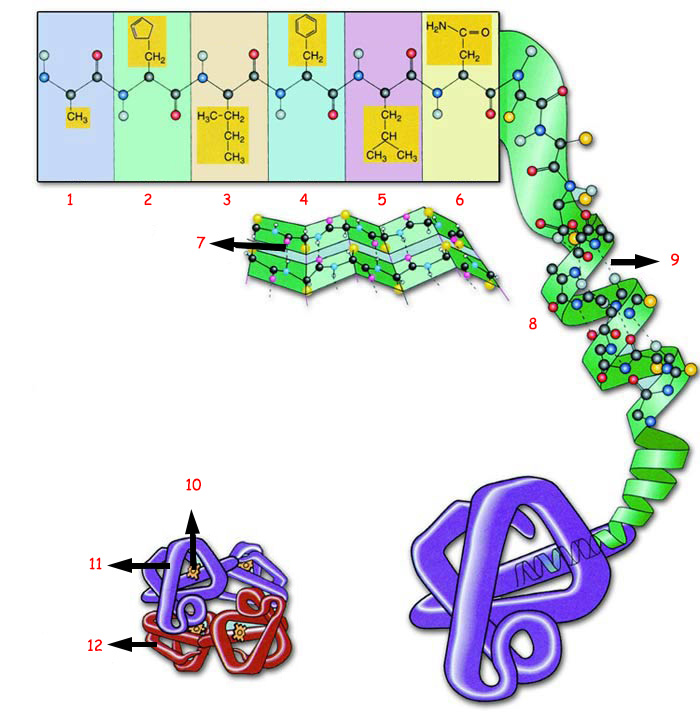 |
|||
|
1. Amino Acid 1 |
4. Amino Acid 4 |
7. Hydrogen Bond |
10. Heme Group |
|
A. PRIMARY STRUCTURE: B. SECONDARY STRUCTURE: C. TERTIARY STRUCTURE: D. QUADERNARY STRUCTURE: |
|||
|
The proteins' physical, chemical and biological properties and the resulting functions they will perform—determine the structures of the amino acids that comprise them, as shown in the diagram. |
|||
Of course, you can find similar information about protein structure in any biology or biochemistry text. The reason why we consider these matters here is to show how truly complex and interrelated are the structures, effects and systems that give rise to proteins. Darwinists describe the "spontaneous" formation of a protein as if the process were very simple and quite able to accommodate coincidences. Only by concealing the exceedingly complex structure in proteins do they hope to make the myth of chance convincing. In describing the structure of proteins, therefore, they imply that proteins can easily be formed by amino acids binding to one another, like beads on a necklace. In fact, however, as is clear from this account so far, that even if amino acids could combine with one another at random, a number of other conditions need to be fulfilled. In the event that these are not useful, proteins cannot form.
When you read the information that follows, therefore, recall that coincidences cannot make fine planning or calculations, much less bind amino acids to one another with special structures and methods.
Proteins' Primary Structure: Amino Acid Sequence
The most important determinant of proteins' forms, which are exceedingly important for life, is the sequence of the amino acids that constitute them. Abnormalities in amino acid sequences are the cause of many genetic diseases. From that perspective, the correct sequence of amino acids, is of the greatest importance for health.
The amino acid sequence serves like a backbone for proteins, and the backbone, or sequence, of each variety of protein has been created specially for it. Just as the backbone determines the shape of a vertebrate's body, so the sequence of proteins determine their shape. Every amino acid is analogous to a vertebra in that backbone. Just as every vertebra must be in a specific place in order for the body to function, so every amino acid must be in a specific position for proteins to display certain properties. Though the functions carried out by the "spine" in proteins are similar to those in our bodies, there is one important difference: Protein backbones operate in an area of just one millionth of a millimeter. No doubt, a structure able to operate an important function in such a small space is most miraculous.
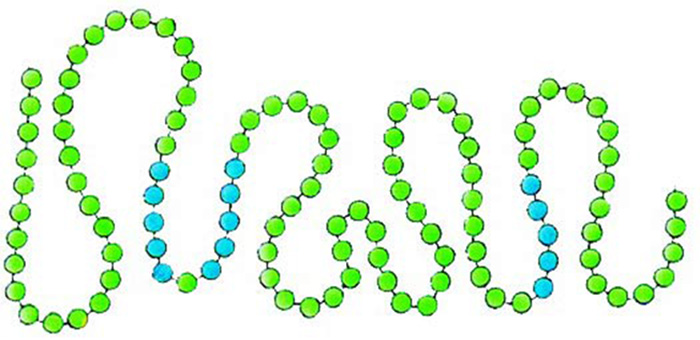 |
|
The proteins' primary structures emerge with the amino acids being strung out like beads on a necklace. |
Just like the spine and vertebrae in your own body, proteins and amino acids have been specially created to attach to one another in the best possible manner. Their flawless attachment is just as important to proteins as it is to the body. If one amino acid does not bind to the next in an appropriate sequence, then the entire protein loses its function. Reflect a little, and you can discern the delicate and conscious creation here.
Miraculous events take place constantly inside all the 100 trillion cells in the human body. In an area of one thousandth of a millimeter, too small to be seen with the naked eye, thousands of proteins comprising the cell, and the hundreds of amino acids that form these proteins, are all in exactly the right positions. That applies to all the billions of human beings on Earth. Contrary to what Darwinists would have you believe, this extraordinary phenomenon is not the work of chance. In addition, never forget that amino acids are not conscious entities with sensory organs and the ability to think, but tiny molecules made up of specific combinations of unconscious atoms. That being so, Who is it Who decides how the proteins necessary for life will come about, and which amino acid are to bind where? Could the various atoms have come to a joint decision one day and said "Let us combine in a particular order and make up an amino acid. Then let us agree with other atoms com prising other amino acids to arrange ourselves in a particular sequence to produce a protein"? Of course, such a claim would be utterly illogical.
Just as unconscious atoms can possess no such ability, neither can proteins or the amino acids that compose them possess any such decision-making mechanism. Allah locates all these entities in the appropriate positions, brings the building blocks of living cells into being, and creates life—flawless and of infinite variety—by means of these cells. Allah is Lord of all the worlds, from atoms to giant galaxies.
Proteins' Secondary Structure: Helix and Layered Structure
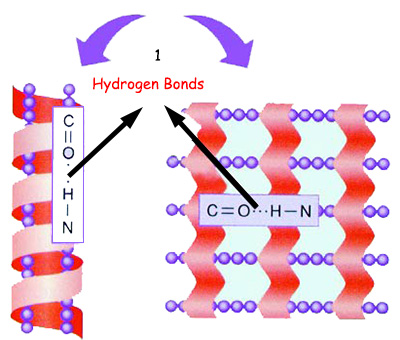 |
|
1. Hydrogen bonds |
|
When amino acids are bound by hydrogen bonds as well as to peptide bonds, the protein chain assumes a helix or layered form, known as the protein's secondary structure. |
After the amino acids necessary for a protein line up alongside one another, other miraculous events take place. Along with the peptide bond that every amino acid sets up with the amino acid next to it, hydrogen bonds also form. How these bonds form determines the shape and position that amino acids will assume along the sequence.
Under some circumstances—for instance, when hydrogen bonds form within the chain—the amino acid forms a spiral structure. When amino acids establish weak bonds with an amino acid outside that chain, then layered structures form, reminiscent of the steps on a staircase.
Proteins whose chains assume a spiral form resemble the springs in mattress or automobile seat and, just like them they twist around a central axis. The proteins in hair, and myosin, a protein in muscles, possess this spiral structure and as a result, are elastic because hydrogen bonds can easily break and reform just as easily.
 |
|
The picture to the side shows the structure of myosin, a muscle protein with a secondary structure. Myosin has a spiral structure and is therefore elastic, and the hydrogen bonds formed between the amino acids can be broken. |
The discovery of the effects of hydrogen bonds on body proteins has resulted in various applications in daily life. For example, to straighten curly hair or put curls into straight hair, the hydrogen bonds between the amino acids in hair proteins must be broken and reconstituted. 11
Proteins in layered form with a secondary staircase structure are not as flexible as those arranged in a spiral structure. They do, however, permit the formation of structures that bend, one very important requirement of living things. For example, proteins like the silk fibers in cocoons and spider webs are set out parallel and form chains bound to one another with hydrogen bonds. Because the peptide atoms are bound perpendicularly to the protein chain, the spine of these proteins bends up and down like a strand of yarn. 12
In living things, the folds in proteins are always exactly where they need to be. If fibroins, the proteins in spider webs, lacked the ability to bend, then the webs would serve no purpose. But this protein's structure provides the web with a resilience that keeps prey from escaping. And spider silk is five times stronger than steel of the same thickness (1/1,000th of a millimeter in diameter). 13
As you see, proteins' structures have been created flawlessly and incomparably for the survival of living things, right down to the finest detail. Even if all the atoms in the universe were placed at its disposal, blind coincidence could never operate with such foresight and perform such impeccable calculations. No chain of atoms that comes into being by chance can possess the information, intellect or ability to organize every atom in such a way that the spider web becomes most efficient.
 |
|
|
The picture to the side shows the structure of myosin, a muscle protein with a secondary structure. Myosin has a spiral structure and is therefore elastic, and the hydrogen bonds formed between the amino acids can be broken. Below is shown the three-dimensional structure of silk fibroins. The proteins in silkworm cocoon fibers and spider webs are set out parallel to one another, consisting of chains bound to one another with hydrogen bonds, making them straight and pliable. |
|
|
1. Ala |
2. Gly |
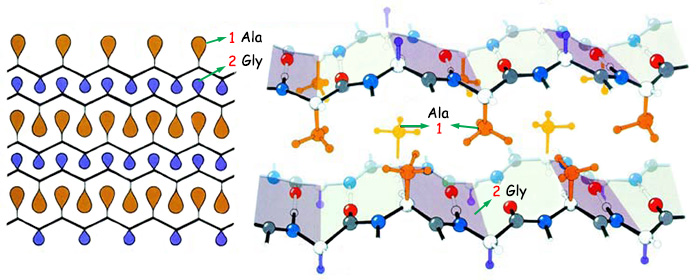 |
|
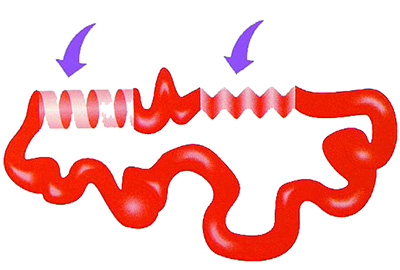
The Tertiary Structure of Proteins
 |
|
After proteins as sume their sec ond ary structures, they adopt new shapes by bending, fold ing and mak ing sudden turns. In this way the ter ti ary structure emerges. |
After assuming the forms in their secondary structures, proteins begin to assume new shapes by bending, folding, or even making sudden U-turns under the influence of amino acids that approach or move away from one another. This bending and folding is enabled by the mutual effects between amino acids' side chains. In this way emerge three-dimensional forms of great functionality. So how does this bending process, the result of these mutual effects, occur?
In proteins, the side chains of amino acids attract or repel one another as a result of various influences. Five major agents play a role in this repulsion and attraction: hydrogen bonds, disulphide bonds, ionic bonds, Van der Wallis forces and other polar and non-polar effects of the side chains.
By means of these special bonds, some sections of amino acids draw closer to one another. The amino acid chain folds over itself. Proteins bend at the appropriate sites and angles. The three-dimensional form of the protein is stabilized and kept from dissolving in the extracellular environment.
Experiments have shown these bonds to be of crucial importance. Every one of them permits the protein molecule to bend in exactly the desired manner in various sites along its length. For example, disulphide bonds form only in specific regions of the protein molecule, but permit a particular bending in those regions and to the exact extent required. In a similar way, other forces act on amino acid regions to cause certain sections of the chain to approach one another, or to move away. The absence of any one of these necessary folds and curves will render the protein useless.
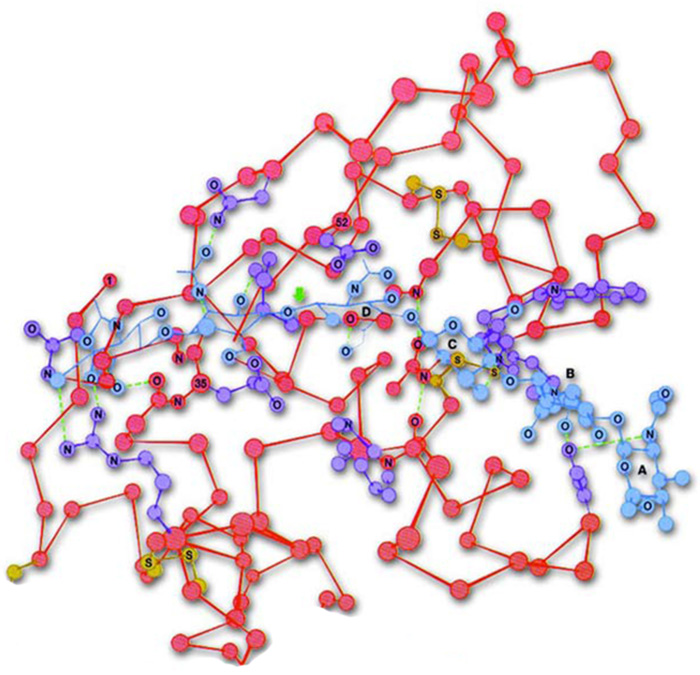 |
|
Three-dimensional form of the protein lysosome |
The Strength of the Bonds Must Be Ideal
These bonds essential to protein formation are different from other powerful bonds. Proteins' curved three-dimensional forms cannot arise through other powerful chemical forces because the strength of the bond formed would cause the molecules to approach one another too closely and thus cause the protein to lose its properties. Therefore, these bonds whose features and strengths have already been identified are at the ideal strength to let the proteins to bend.
Through these bonds, the protein process is also speeded up. As the well-known biologist James D. Watson explains:
Enzyme-substrate complexes can be both made and broken apart rapidly as a result of random thermal movement. This fact explains why enzymes can function so quickly, sometimes as often as 106 times per second. If enzymes were bound to their substrates by more powerful bonds, they would act much more slowly. 14
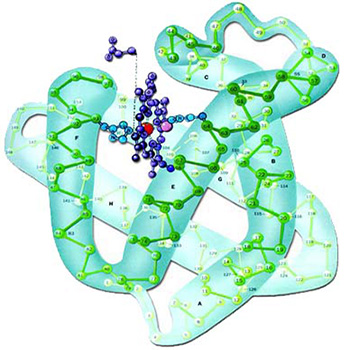 |
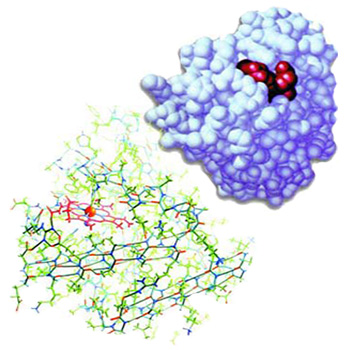 |
|
The three-dimensional structure of the protein myoglobin, shown to reveal its complexity. It is impossible for a perfect structure able to fulfill such important functions to have come into being by chance. |
The picture to theside shows the three-dimensional form of the protein myoglobin and the peptide groups among the atoms. |
Proteins' Three-Dimensional Structure is a Flawless Creation
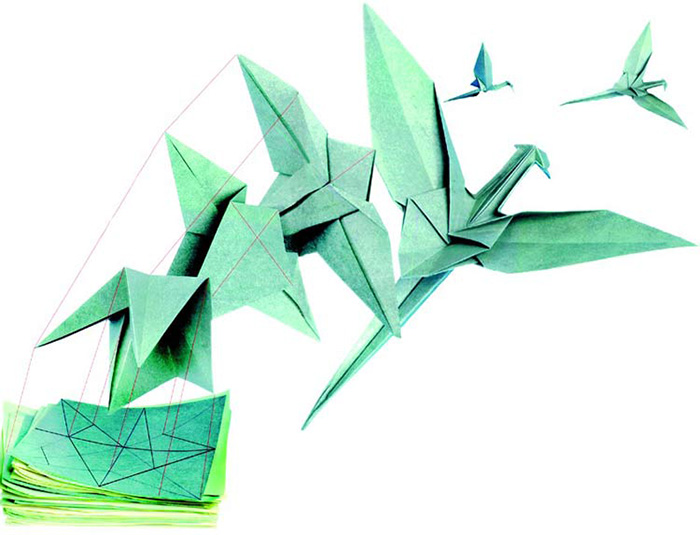
To dramatize the bending of the protein chain in its timing, location, direction and angle, consider the Japanese art of origami, or paper folding. In order to obtain a three-dimensional "sculpture," a two-dimensional piece of paper is subjected to consecutive creasing and folding operations. By following predetermined instructions, you can fold a flat, rectangular sheet of copier paper into a model of a ship or a bird. In much the same way, for a protein to assume a three-dimensional form, its amino acid chain must fold at specific intervals and specific angles, in specific lengths and directions.
In origami, it is impossible to obtain the three dimensional forms by random folding. For every model that will be obtained, experts have designed in advance which part of the paper is to be folded in which order and in which way. A single fold out of sequence, in the wrong direction or the wrong length will prevent the desired shape from emerging, and the resulting form will be defective and impaired. (For instance, miss out one fold while making a paper airplane, and the plane's wing will fail to emerge at the proper angle, due to that single faulty fold.)
When it comes to proteins, however, the situation is far more detailed. One single sequence error or faulty combination in just one amino acid will cause the protein molecule to assume a faulty shape that will not function. For instance, the spherical shape of the protein myoglobin is responsible for the transport of oxygen in the muscles. When impaired, its length can becomes 20 times greater than its width, and it becomes unable to carry oxygen molecules. 15
 |
|
The bends in the protein chain are the work of a superior creation. If you fold a sheet of paper into the 3-D model of a bird by following the instructions, even one incorrect fold will keep your origami bird from emerging.Naturally, the necessary folds in any protein are much more complex, and could never come about by chance. |
On their own or even together, amino acids cannot undertake vital functions inside the body. But through these folds and curves, they acquire enormous potential, in the same way that a sheet flat piece of paper assumes the shape of a ship or bird through planning, design, and conscious bending and folding. Remember, a protein's structure is a great deal more complex and organized than the most sophisticated origami. Even though the protein molecule is too small to be seen with the naked eye—or even under an electron microscope!--the atoms arranged into such a minute space are first set out according to a planned goal and then bent and folded—again in line with that goal All these features are far more extraordinary and astonishing than in any arrangement you may see around you.
In these most minute building blocks of life there is absolutely no room for chance formation. For such a flawless, complex, multi-stage and multi-component structure in order to come into being by chance is manifestly impossible. Moreover, this description is merely a simplified summary of the countless details regarding proteins' structure. More detailed investigations reveal still more complex features of these protein molecules, and a great many questions have still not been fully answered to this day.
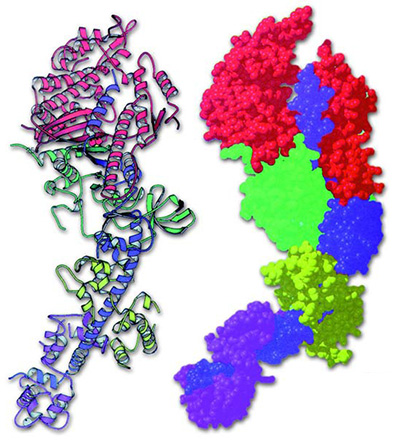
The Quaternary Structure of Proteins: Combined Proteins
 |
|
Proteins combine together by means of complex folds, giving rise to their quaternary structure. |
Imagine a desk with several telephones on it, whose cords all become tangled up with one another. At first sight, it appears impossible to determine which cord belongs to which phone. Proteins, too, also intertwine with one another in very complex ways.
Many proteins become able to perform their functions only after combining with one another. However, in order for proteins to combine into giant molecules, very delicate balances have to be established. If two proteins are to combine, their shapes must be as suited to one another as a hand to a glove. Think of jigsaw puzzles as an example of this essential compatibility. If the curves and extensions of one single piece do not match the next, then completing the picture will be impossible. The same applies to proteins. If the bonds of just one protein is not correct, the giant combined molecule will serve no purpose. 16
Furthermore, if combined proteins are to discharge their functions, it is also essential that they come together in the right numbers. The hormone insulin is an example. This protein organizes the giving of the order to store excess sugar in the bloodstream by the combination of more than one amino acid chain. Any flaw in the insulin molecule's structure will make it useless and cause the individual to suffer from diabetes. When insulin fails to function, the sugars that enter the bloodstream are excreted without being fully metabolized or stored against future need. As a result there can be insufficient sugar in the blood, and the cells' energy requirements are not met. In such a situation, weakness and even death are inevitable.
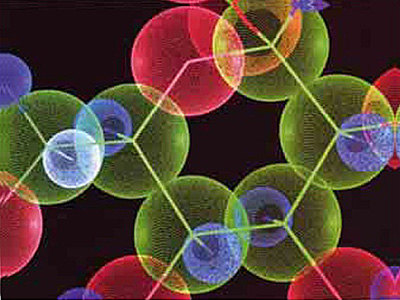 |
|
Insulin molecule |
Similarly, there must not be a single error in the structure or form of any single protein in any of the 200 or so types of cell in your body. Every stage of this formation is planned and acted upon according to the last stage in it, in other words, the target information. Only when the hormone adrenalin—a protein secreted by the adrenal glands—has the correct structure can the heart and muscle cells recognize it and be stimulated into action, to protect the body against physical and psychological stress. In the same way, all the enzyme proteins in our bodies can carry out their functions—such as cell division, energy production, molecule transport and a great many more—only by means of the shape they possess.
Biochemists use modern-day technology to research these molecules of life. Every new piece of amazing formation they obtain has revealed this incomparable creation even further, and demonstrated the illogicality of claims that chance could "evolve" such a system. By a most defective logic, Darwinists believe in coincidences as a creative deity and claim that structures with such a complex and superior system came into being as a result of chance. Only sincere, rational individuals of good conscience are able to see the truth, Allah reveals us in the Qur'an:
 |
|
For proteins to combine and produce giant molecules, they must be compatible with one another and be able to fit like the pieces of a jigsaw. |
Allah is One God.
There is no god but Him, the All-Merciful, the Most Merciful.
(Surat al-Baqara: 163)
Footnotes
3.Prof. Dr. Ali Demirsoy, Kalitim ve Evrim ("Inheritance and Evolution"), Ankara:Meteksan Publishing Co., 1984, p. 61 (emphasis added). 
5.Fabbri Britannica Science Encyclopedia, Vol. 2, No. 22, p. 519. 
6.Vance Ferrell, Dna, Protein and Cells, Harvestime Books, 1996, p. 24. 
7.Walter T. Brown, In the Beginning: Compelling Evidence for Creation and the Flood (7th Edition); http://www.creationscience.com/onlinebook/LifeSciences40.html 
8.Prof. Dr. Engin Gozukara, Inonu University Medical Faculty, Department of Biochemistry, Biochemistry, Nobel T›p Kitabevleri 1997, 3rd Edition, Vol. 1. pp. 123-124. 
9.P.A.Temussi, et al., "Structural Characterization of Prebiotic Polypeptides," Journal of Molecular Evolution 7, (1976): 105. 
10. Mere Creation: Science, Faith and Intelligent Design, Edited by William A. Dembski, Intervarsity Press, Illinois, 1998, pp. 125-126. 
11.Curtis Barnes, Invitation to Biology, New York: Worth Publishers, Inc., 1985, p. 49. 
12.Michael Behe, Darwin's Black Box, New York: The Free Press, 1996, p. 264. 
13."Structure and Properties of Spider Silk," Endeavour, January 1986, Vol. 10, p. 42. 
14.James Watson, The Molecular Biology of the Gene, New York: W. A. Benjamin, Inc., 1965, p. 126. 
3 / total 7
You can read Harun Yahya's book The Miracle of Protein online, share it on social networks such as Facebook and Twitter, download it to your computer, use it in your homework and theses, and publish, copy or reproduce it on your own web sites or blogs without paying any copyright fee, so long as you acknowledge this site as the reference.